+86-15212299029
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
Views: 220 Author: Site Editor Publish Time: 2025-11-14 Origin: Site











Content Menu
● Visual Representation of Phosphate
● The Role of Phosphate in Nature
● Industrial Applications of Phosphate
>> Fertilizers
Phosphate is a vital chemical compound that plays a crucial role in various biological and chemical processes. Understanding its structure, appearance, and significance can provide insights into its importance in both nature and industry. This article will explore what phosphate looks like, its molecular structure, its various forms, and its applications.

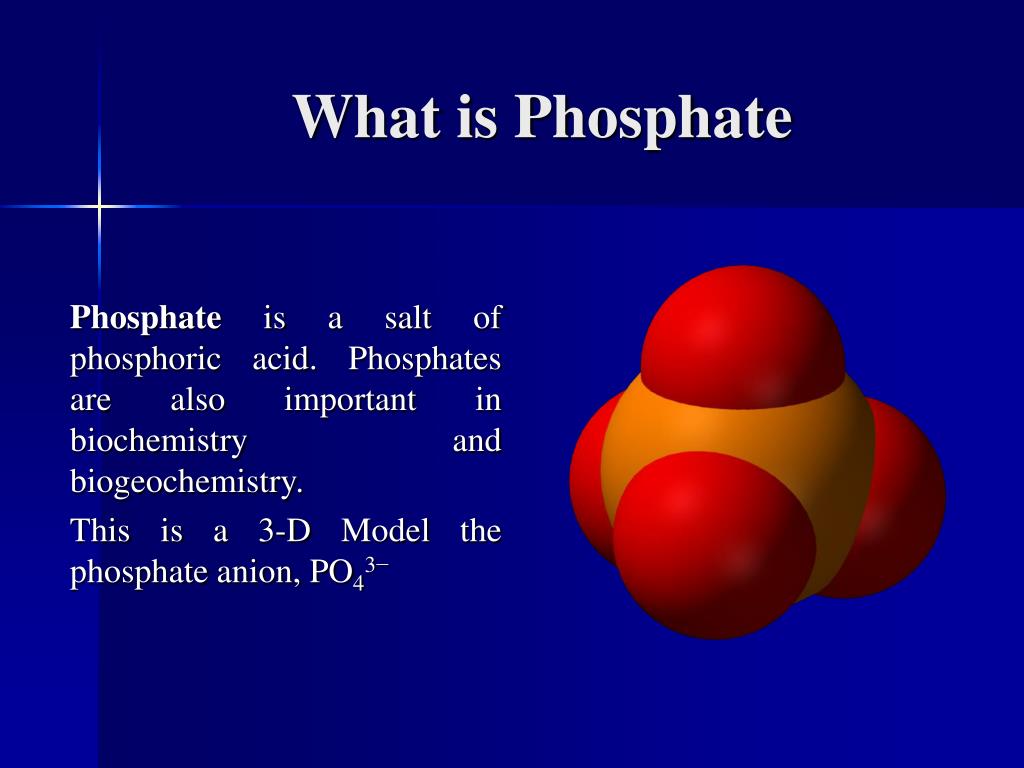
Phosphate refers to a chemical compound that contains the phosphate ion (PO₄⊃3;⁻). This ion consists of one phosphorus atom covalently bonded to four oxygen atoms, forming a tetrahedral shape. The phosphate ion is a key component in many biological molecules, including DNA, RNA, and ATP (adenosine triphosphate), which is essential for energy transfer in cells.
The phosphate ion has a tetrahedral geometry, which means that the four oxygen atoms are positioned at the corners of a tetrahedron with the phosphorus atom at the center. This arrangement allows for the formation of various compounds, including phosphates and phosphoric acid. The phosphate ion carries a negative charge, which is crucial for its reactivity and interactions with other molecules.
Phosphate can be represented in various ways, including:
- Ball-and-Stick Model: This model visually depicts the phosphate ion, showing the phosphorus atom at the center with four oxygen atoms surrounding it. The bonds between the atoms are represented by sticks, while the atoms themselves are represented by balls of different colors. Typically, phosphorus is shown in orange, and oxygen in red.
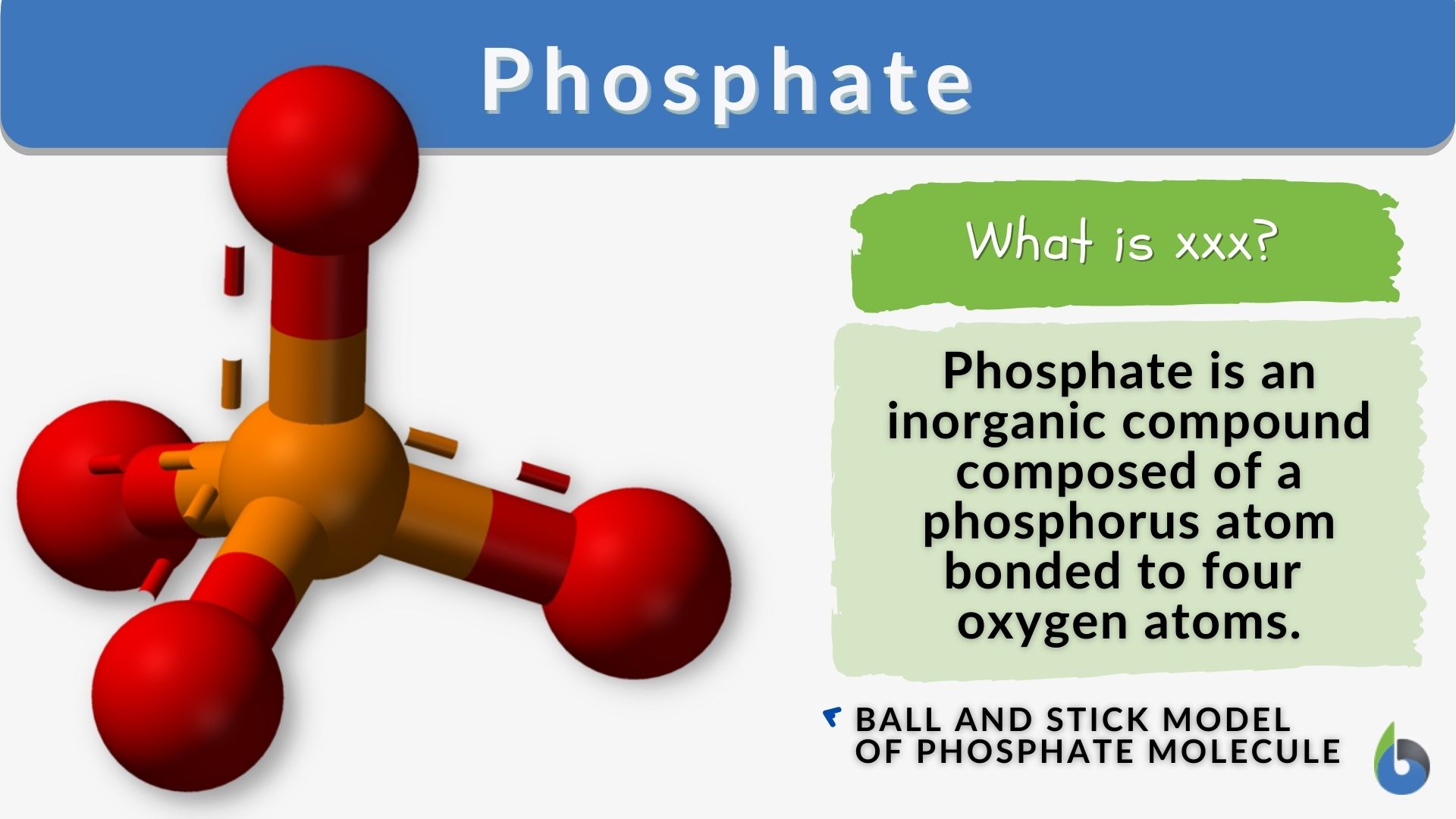
- 3D Structural Models: These models provide a more detailed view of the spatial arrangement of atoms in the phosphate ion. They can illustrate the bond angles and distances between atoms, which are important for understanding the chemical behavior of phosphates.
Phosphate itself, in its pure ionic form, is not something that can be seen directly. However, when it is part of various compounds, its appearance can vary:
- White Phosphorus: This is a waxy, white-yellow solid that is highly reactive and can glow in the dark due to its luminescence. It is important to note that white phosphorus is toxic and can cause severe burns upon contact with skin.
- Red Phosphorus: This form is an amorphous, non-toxic solid that is used in safety matches and fireworks. It appears as a dark red or purple powder.
- Black Phosphorus: This is the least reactive allotrope of phosphorus and resembles graphite in appearance. It is a black, flaky solid that conducts electricity.
Phosphate is essential for life. It is a key component of DNA and RNA, which are critical for genetic information storage and transfer. Additionally, phosphate groups are involved in energy transfer through ATP, which powers cellular processes.
Phosphates are also important in ecosystems. They are a major component of fertilizers, which are used to enhance plant growth. However, excessive phosphate runoff into water bodies can lead to eutrophication, causing harmful algal blooms and disrupting aquatic ecosystems.
Phosphates are widely used in agriculture as fertilizers. They provide essential nutrients to plants, promoting healthy growth and increasing crop yields. Common phosphate fertilizers include monoammonium phosphate (MAP) and diammonium phosphate (DAP).
Phosphates are used as food additives to enhance flavor, preserve freshness, and improve texture. They are commonly found in processed foods, dairy products, and meats.
In the pharmaceutical industry, phosphates are used in various medications and supplements. They play a role in the formulation of drugs and are essential for certain biochemical processes in the body.
Phosphate is a versatile and essential compound that plays a significant role in both biological and industrial contexts. Its unique tetrahedral structure and various forms make it a critical component in many processes, from energy transfer in cells to agricultural applications. Understanding what phosphate looks like and its importance can help us appreciate its role in our lives and the environment.

1. What is the chemical formula of phosphate?
- The chemical formula of phosphate is PO₄⊃3;⁻, indicating one phosphorus atom and four oxygen atoms.
2. What are the different forms of phosphorus?
- The main forms of phosphorus include white phosphorus, red phosphorus, and black phosphorus, each with distinct properties and appearances.
3. Why is phosphate important for plants?
- Phosphate is crucial for plant growth as it aids in energy transfer, photosynthesis, and the synthesis of nucleic acids.
4. How does phosphate affect water quality?
- Excessive phosphate runoff can lead to eutrophication, causing algal blooms that deplete oxygen in water bodies and harm aquatic life.
5. What are some common uses of phosphates in food?
- Phosphates are used as preservatives, flavor enhancers, and texture modifiers in various processed foods.
Hot Tags: China, Global, OEM, private label, manufacturers, factory, suppliers, manufacturing company



